Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Inbal Israely, PhD

Inbal Israely, PhD
While information can be stored in the brain over our entire lifespan, it is unclear how this information is physically encoded and how the fidelity of connections is maintained. The focus of research in my laboratory is grounded in the guiding principle that neuronal structure and function are intimately linked, and we aim to determine how this relationship allows our brains to learn and remember. Specifically, we are interested in understanding how synaptic activity can lead to specific structural changes, how such changes affect connectivity within neural circuits, and how these events are important for learning and memory.
In the lab, we focus on single neurons, even single spines, to understand the cellular mechanisms that are important for structural plasticity. Does activity arriving at one or multiple inputs become encoded in the same way? Are some forms of activity more efficacious than others, and do they lead to long lasting changes of spines? We can begin to understand this by tracking the outputs of synaptic plasticity in real-time, since individual spines physically change in size as their synapses change in efficacy. Using 2-photon microscopy, we stimulate living spines (through the activation of caged glutamate) and then visualize structural changes (both in size and shape), while monitoring electrophysiological responses or calcium signals using genetically encoded fluorescent reporters.
Some of the specific questions that my lab is currently investigating include: 1) How does new protein synthesis contribute to structural plasticity and synaptic organization of single and groups of synapses, and how does dysregulation of this process contribute to human cognitive dysfunction; 2) How do synapses integrate diverse patterns of information, are there specific calcium signatures associated with this activity, and what are the long term structural correlates of these events; 3) When different forms of plasticity overlap, what are the functional and structural consequences for specific inputs?
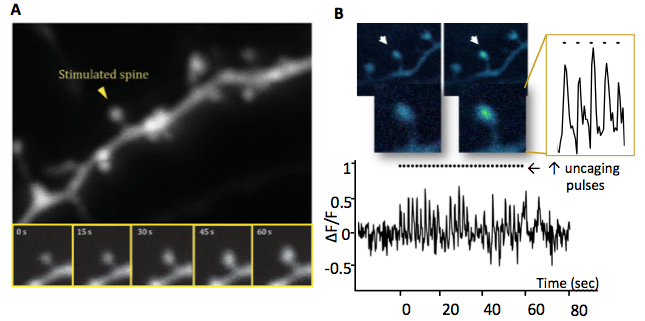 Figure 1: Long term potentiation of a single synapse. Activity on a spine leads to growth and an influx of
calcium within the head which can be optically detected. A. Glutamate uncaging mediated synaptic
potentiation and spine enlargement (time lapse images below). B. A neuron labeled with GCaMP
allows visualization of the calcium signal in the spine head in real time during glutamate stimulation.
|
By stimulating individual synapses and monitoring the induced changes in plasticity and structure over many hours, we have demonstrated that activating one spine can facilitate the growth of a nearby neighbor that is stimulated later if new proteins are made (Govindarajan*, Israely* et al., 2011). If several spines are stimulated at the same time, such activity leads to competition for growth and new proteins, and results in changes in spine volume. In addition to synaptic interactions during potentiation, we are investigating the structural consequences of protein synthesis dependent long term depression, activated by metabotropic glutamate receptors (mGluRs). We have shown that inducing mGluR-dependent LTD leads to robust spine shrinkage that lasts for many hours, and even spine elimination (Cortes & Israely, 2013). Protein synthesis dependent synaptic depression, like potentiation, leads to increased protein availability in dendrites and thus may impact competition for plasticity between inputs. We have observed that spines undergo a wide range of physical changes in response to activity, and that these features fluctuate over time. In collaboration with colleagues whose expertise lies in complex medical image analysis, we developed a semi-automatic structural analysis toolbox that allows for the quantification of a variety of different structural features in neurons over time (Ghani et al., 2017).
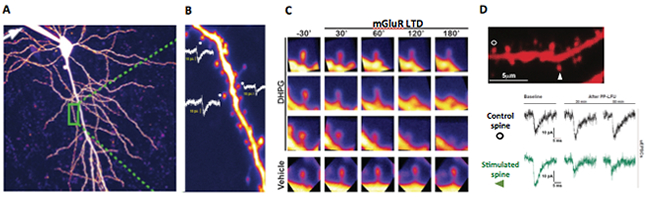 Figure 2: Long term depression and structural plasticity at single spines. A. Visualization of living single spines in a CA1 hippocampal pyramidal neuron with 2-photon microscopy. B. Patch clamp mediated fluorescent labelling and electrophysiological recordings of single synapse responses to uncaging evoked currents and their size dependent correlation with spine volume. C. Visualization of spine shrinkage upon mGluR-mediated synaptic depression with the agonist DHPG. D. Single spine mGluR-dependent synaptic depression via glutamate uncaging.
|
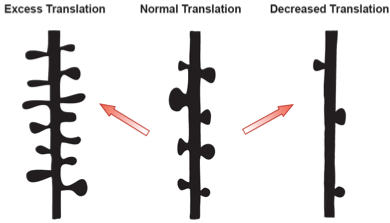
Figure 3: Protein translation dependent synaptic organization. Schematic representation of a dendritic branch with spine density changes due to alterations in new protein availability. The middle represents a normal spine distribution, while excess or too few proteins will lead to structural abnormalities similar to those observed in human disorders.
Together, these studies are contributing to our understanding of how synaptic activity physically shapes dendrites. We hypothesize that the appropriate level of cellular proteins during learning and information recall contributes to the structural and functional integrity of circuits that are critical for healthy neuronal function (Ramiro-Cortes et al., 2013). Perturbations of this system, leading to either too many synaptic inputs or too few, could contribute to cognitive deficits. We are currently examining whether alterations of protein synthesis pathways affect synaptic cooperation and competition under these conditions, and use animal models of human disorders to probe for common principles of neuronal dysfunction. Thus, by combining molecular and genetic tools together with imaging and electrophysiological methodologies, we study how information is physically stored in the brain. Through this approach, we hope to learn how neurons process information in a state of health, as well as to unravel what can go wrong during disease.
 From left to right, members of the Israely lab include Alexandra Panzarino and Dr. Inbal Israely (PI), as well as Maria Royo and Weiqun Fang (not pictured).
|
Inbal Israely, PhD
Assistant Professor of Pathology and Cell Biology (in Neuroscience and the Taub Institute)
ii2176@cumc.columbia.edu