Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
April 2017
#2 Age-Related Biomarkers in LLFS Families With Exceptional Cognitive Abilities
Brain Atrophy Can Introduce Age-Related Differences in BOLD Response
 Qolamreza R. Razlighi, PhD
Qolamreza R. Razlighi, PhDThis project attempts to address one of the longstanding and key issues in the studies of brain aging using functional magnetic resonance imaging (fMRI). Use of fMRI in studies of aging is often hampered by uncertainty about age-related differences in the actual neuronal activity or in the amplitude of the hemodynamic response. Such uncertainty introduces a significant challenge in the interpretation of the fMRI results, making it almost impossible to disentangle whether or not the observed age-related differences are due to the differences in the underlying neuronal activity or due to age-related differences in hemodynamic response. Even though this issue has been extensively investigated in the field of neuroimaging, there is currently no consensus about the existence and potential sources of age-related hemodynamic alterations. Results from existing studies often contradict each other, making it extremely difficult to draw any conclusion.
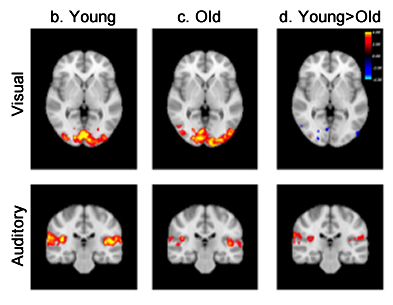
Figure 1. Brain activation induced by visual (top row) and auditory (bottom row) stimuli illustrated by color-coded z-statistics overlaid on the MNI template for (b) young subjects, (c) old subjects, and (d) the contrast between young and old subjects.
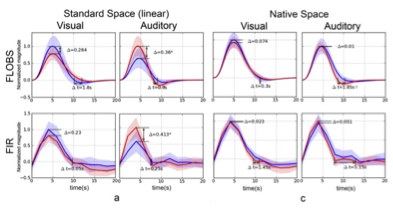
Figure 2. Differences between extracted HRFs from young (red curves) and old (blue curves) subjects using visual and auditory stimulation in real fMRI data. (a) standard space with linear (affine) registration, and (c) native space analysis without registration. The significant difference in the magnitude is marked with * and in the delay (from peak to baseline) is marked by †(P<0.05, outliers excluded).
In the present study, published online in the journal of Human Brain Mapping, Drs. Qolamreza "Ray" Razlighi, Xueqing Liu (Biomedical Engineering), and colleagues used an event-related fMRI experiment with two robust and well-studied stimuli (visual and auditory) to detect a significant age-related difference in the amplitude of response to auditory stimulus (figure 1). Accounting for brain atrophy by circumventing spatial normalization and processing the data in subjects' native space eliminated these observed differences (figure 2). In addition, Dr. Razlighi and colleagues simulated fMRI data using age differences in brain morphology while controlling hemodynamic impulse response function (HRF) shape. Analyzing these simulated fMRI data using standard image processing resulted in differences in HRF amplitude, which were eliminated when the data were analyzed in subjects' native space. These results indicate that age-related atrophy introduces inaccuracy in co-registration to standard space, which subsequently appears as attenuation in BOLD response amplitude. According to the authors, their findings explain most of the existing contradictory reports regarding age-related differences in the fMRI BOLD responses.
Qolamreza R. Razlighi, PhD
Assistant Professor of Neuroimaging (in Neurology and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
Adjunct Assistant Professor in Biomedical Engineering
Quantitative Neuroimaging Lab
qr2108@cumc.columbia.edu
Age-Related Biomarkers in LLFS Families With Exceptional Cognitive Abilities
 |  | |
| Sandra Barral, PhD | Nicole Schupf, PhD |
The factors that characterize successful aging and exceptional longevity are not fully established. As the proportion of elderly adults in the population grows, predictors of exceptional longevity become increasingly important to allow the development of approaches to promote healthy aging. Exceptional longevity can be defined in a number of ways, including survival to a specific extreme age (longevity), disability-free (active life expectancy), disease-free (healthy aging), or cognitively intact survival, and studies have also demonstrated a strong familial component to exceptional longevity.
Drs. Sandra Barral, Nicole Schupf, and colleagues in the Long Life Family Study (LLFS) have been studying the role of cognition in exceptional longevity in a cohort of multigenerational families in the United States and Denmark, where long-lived individuals, their siblings, and their offspring were recruited for an examination that characterized key intermediate phenotypes of longevity, including major chronic diseases, risk factors, physical and cognitive function, blood-based and genetic biomarkers.
Studies in diverse elderly populations have consistently reported that age-related cognitive impairment is associated with higher risk of mortality, even after adjustment for a variety of health conditions, lifestyle factors, and socio-demographic characteristics. Results from the LLFS have consistently suggested that preservation of cognitive function is a key feature of exceptional longevity. Previous work by Dr. Barral and colleagues found that offspring of probands from the LLFS showed better cognitive performance on multiple cognitive tasks compared with individuals without a family history of longevity, and showed that exceptional episodic memory performance strongly aggregates in the LLFS families and may be genetically modulated. In this study, published in the Journals of Gerontology: Medical Sciences, Barral et al. investigated whether LLFS families with exceptional cognition may also show more favorable profiles of other age-related biomarkers.
Families were categorized as showing exceptional cognition if two or more offspring of exceptionally long lived probands scored high on a composite set of cognitive tasks. Then, families with exceptional cognition were compared with families without exceptional cognition for longevity and for 28 traits from 5 health-related domains (cognitive, cardiovascular, metabolic, physical, and pulmonary). Families with exceptional cognition showed significantly higher family longevity and had a significantly better metabolic/cardiovascular profile than those of LLFS participants from non-exceptional cognition families. The healthier metabolic profile was related to obesity in an age-dependent fashion. The prevalence of obesity in families with exceptional cognition was significantly lower compared with families without exceptional cognition (38% vs 51%, p = .015) among family members less than 80 years of age. However, among members of families with exceptional cognition 80 years of age and older, the prevalence of obesity was higher (40% vs 38%, p = .011). These findings are consistent with previous work showing a broadly consistent protective association of obesity with cognitive function in late-life, that is, overweight and obese elderly subjects are at lower risk of cognitive impairment. Families with exceptional cognition also showed better physical/pulmonary function than families without exceptional cognition (β = 0.51, SE = 0.25, p = .042). Thus, exceptional longevity, like other complex traits, appears to be a multidimensional phenotype, that likely includes multiple domains such as cognition, metabolic, physical/pulmonary, and cardiovascular traits, each of them measuring multiple and correlated indicators of healthy aging.
Sandra Barral, PhD
Assistant Professor of Neurogenetics (in Neurology, the Gertrude H. Sergievsky Center, and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain) at CUMC
smb2174@cumc.columbia.edu
Nicole Schupf, PhD
Professor of Epidemiology (in Neurology, Psychiatry, the Gertrude H. Sergievsky Center, and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
ns24@cumc.columbia.edu