Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
January 2015
A novel role for microRNA-219 in Alzheimer’s disease and tauopathies

Ismael Santa-Maria, PhD
Alzheimer's disease (AD) and a number of other neurodegenerative diseases are characterized by large quantities of neurofibrillary tangles that build up in brain cells. Tangles are primarily made up of abnormal forms of a protein called tau, but how and why this protein accumulates to such a massive degree in these disorders remains a mystery. Strategies to prevent the formation of tangles have become increasingly sought after for developing AD therapeutics.
Published this January in the Journal of Clinical Investigation, a team of Taub Institute investigators, including Ismael Santa-Maria, Brian McCabe, and John Crary (now at Mount Sinai), found that a specific microRNA, miR-219, which belongs to a class of small RNA molecules that are powerful regulators of gene expression, plays a vital role in a regulatory mechanism that prevents the accumulation of tau protein.
Screening human brain tissue from Columbia's New York Brain Bank, the investigators found miRNA-219 to be markedly downregulated in the brain tissue of patients with AD and severe age-related tauopathy. Their experiments in cell culture systems, and in vivo in a Drosophila model, showed that microRNA-219 function turned off tau expression through a direct interaction with the tau messenger RNA. Further, they found that genetically modified fruit flies that express human tau are protected from neurodegeneration by increasing the levels of microRNA-219, while toxicity is exacerbated by loss of miR-219.
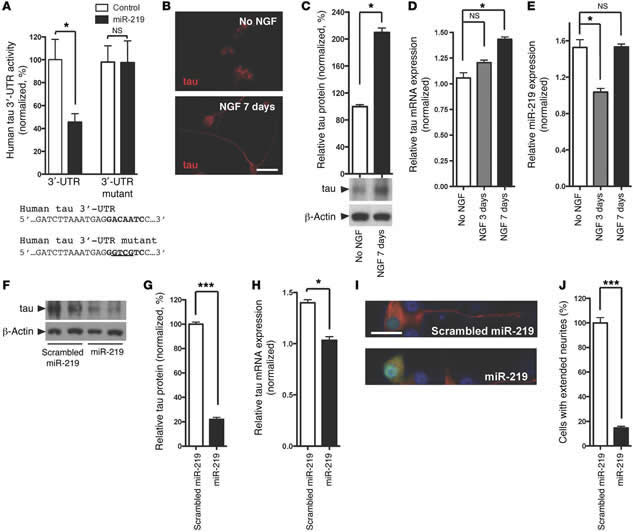 Figure 3 miR-219 directly regulates tau expression and attenuates neurite outgrowth in mammalian cell cultures. (A) Cotransfection of human neuroblastoma cells (SH-SY5Y) with a miR-219 mimic and a dual-luciferase human tau 3′-UTR reporter demonstrated reduced expression compared with that seen in the scrambled miRNA control. Mutagenesis of the miR-219 recognition element abrogated silencing. (B) Immunofluorescence on rat pheochromocytoma (PC12) cells using antisera targeting total tau (tauC) showed extension of processes following treatment with 100 nM NGF. Scale bar: 25 μm. (C) Immunoblot using tauC demonstrated increased tau protein levels following NGF treatment. (D) An increase in tau mRNA levels was observed following NGF treatment. (E) A transient decrease in miR-219 levels (normalized to SNORD24) occurred 3 days after NGF treatment and returned to baseline levels by day 7. (F and G) Quantitative immunoblot analysis using PC12 cells transduced with the lentiviral miR-219 vector revealed reduced tau protein levels compared with those detected in the scrambled miRNA control when differentiated for 7 days. (H) qPCR showed reduced tau mRNA levels in PC12 cells transduced with the lentiviral miR-219 vector. (I) Immunofluorescence microscopy using tauC (red) showed neurite outgrowth in untransduced PC12 cells, but cells transduced with lentiviral miR-219 (green) failed to extend neurites, as judged by the merged image (lower panel; yellow). (J) Quantification of neurite extension revealed a significant decrease in the numbers of tau-positive neurites in cells transduced with miR-219 compared with that observed in untransduced cells. Data are representative of 3 experiments. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 by 2-tailed Student's t test. |
Together, their data indicate that silencing of tau by miR-219 is an ancient regulatory mechanism that may become perturbed during AD, aging, and perhaps other tauopathies. These exciting findings suggest that this regulatory pathway may be useful for developing therapeutics that decrease the production of tau using microRNAs to protect against neurodegeneration. Santa Maria et al. feel they are just beginning to scratch the surface on how microRNAs might contribute to AD. Moving forward, the investigators seek to understand the entire network of genes that these microRNAs control, and whether they can be used therapeutically to halt the progression of these terrible diseases.
Ismael Santa-Maria, PhD
Assistant Professor of Pathology & Cell Biology (in the Taub Institute)
is2395@cumc.columbia.edu
Olfactory Deficits Predict Cognitive Decline and Alzheimer Dementia in an Urban Community

Davangere P. Devanand, MBBS, MD
In the context of the difficulty in developing therapies for Alzheimer's disease (AD) after the clinical diagnosis is made, it has become important to develop methods to detect AD at early or even preclinical stages at which potential treatments may be most effective. Smell (or olfactory) testing provides a simple, noninvasive approach. The underlying mechanism is that olfactory regions, including the olfactory bulb, are infiltrated by neurofibrilllary tangle pathology early in the disease, and AD pathology is present in neuronal pathways projecting from the olfactory bulb and tract to the piriform cortex and medial temporal lobe that are also affected early during the course of AD.
Impairment in odor identification, which relies on odor memory and odor naming, has been shown to be associated with the transition from mild cognitive impairment (MCI) to dementia, particularly in AD. Less information has been available on the predictive utility of odor identification deficits for future cognitive decline in cognitively intact older adults.Â
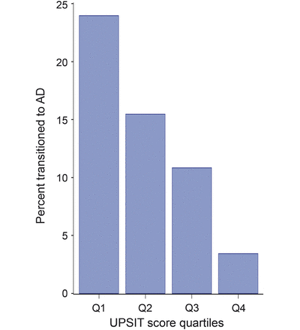
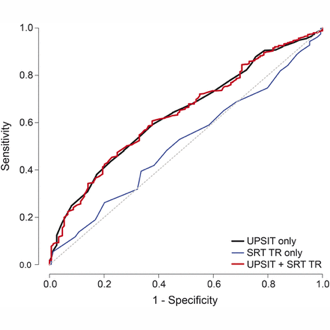
In a study published in Neurology, Dr. D. P. Devanand and colleagues evaluated 1,037 participants without dementia from the Washington Heights/Inwood Columbia Aging Progect (WHICAP) cohort with the 40-item University of Pennsylvania Smell Identification Test (UPSIT), which is a simple multiple-choice test to identify odors that takes a total of 15-25 minutes to administer. In 757 participants in the community cohort, follow-up occurred at approximately 2 years and 4 years. Baseline UPSIT scores were associated with cognitive decline and remained significant (relative risk 1.065 per point interval; 95% CI 1.034, 1.095; p < 0.0001) after including several covariates. Critically, UPSIT, but not Selective Reminding Test-total immediate recall, predicted cognitive decline in participants without baseline cognitive impairment. During follow-up, 101 participants transitioned to AD dementia. In survival analyses, lower baseline UPSIT scores were associated with transition to AD dementia and remained highly significant (p < 0.0001) after including demographic, cognitive, and functional covariates.
|
|
|
The results showed that impairment in odor identification was superior to deficits in verbal episodic memory in predicting cognitive decline in cognitively intact participants. The findings support the cross-cultural use of a relatively inexpensive odor identification test as an early biomarker of both cognitive decline in cognitively intact older adults and of the transition from MCI to AD. Such testing may have the potential to select/stratify patients in treatment trials of cognitively impaired patients or prevention trials in cognitively intact individuals. More broadly, large scale investigations on the predictive utility of olfactory testing in cognitively normal older individuals at increased risk of AD need to be conducted. One limitation to olfactory testing is that it can be affected by smoking and sinus disease, and it can be abnormal in other disorders like Parkinson’s disease that need to be excluded in such studies and when used clinically.
Previous research has shown that olfactory identification deficits add significant predictive value to cognitive, functional and MRI measures in patients with MCI who subsequently transition to AD (Devanand et al, 2008). The results of this study, supported by strong evidence in the literature (every study that has examined odor identification deficits has found significant differences between AD and controls, and have been associated with the transition from MCI to AD), suggest that an olfactory identification test should be compared to more complex and expensive measures, including MRI brain scans and amyloid PET imaging, to determine relative clinical and practical value for predicting future cognitive decline or the clinical diagnosis of AD. Research in this area is critical for the development of preclinical detection methods and to improve our basic understanding of Alzheimer's disease.
Davangere P. Devanand, MBBS, MD
Professor of Psychiatry (in Neurology and the Gertrude H. Sergievsky Center)
dpd3@columbia.edu

William C. Kreisl, MD
Advances in positron emission tomography (PET) have allowed in vivo detection of new targets for molecular therapy for patients with Alzheimer’s disease (AD). One proposed target is the permeability-glycoprotein (P-gp), an efflux translator protein found at the blood-brain barrier. β–amyloid is a substrate for P-gp and evidence suggests that decreased P-gp activity may result in increased accumulation of amyloid plaque in brain. In a recently published Journal of Nuclear Medicine study, Dr. William Charles Kreisl and members of the Molecular Imaging Branch of the National Institutes of Mental Health Intramural Research Program sought to refine the use of PET imaging with 11C-N-desmethyl-loperamide (11C-dLop) to measure P-gp activity in vivo. 11C-dLop is a substrate for P-gp and, under normal circumstances, is kept out of the brain by the efflux transporter. However, when P-gp function is decreased or absent, 11C-dLop is allowed to enter brain, where it is nonspecifically taken up into lysosomes. Therefore, the amount of PET signal found in brain after injection of 11C-dLop is inversely proportional to the activity of P-gp at the blood-brain barrier.
Early 11C-dLop scans performed on patients with AD showed no difference in PET signal from scans performed on healthy controls. These results suggest that either P-gp activity is not related to AD pathology, or that P-gp has such large spare capacity that small changes in AD patients are not sufficient to cause measureable differences on PET imaging. The latter possibility is supported by the fact that over 50% of P-gp transporters must be inhibited before 11C-dlop is allowed entry into brain. Prior studies had performed PET imaging after infusion of the selective P-gp inhibitor tariquidar to block P-gp enough to allow measurable amounts of radiotracer into brain. However, these studies required large doses of tariquidar (greater than 3 mg/kg IV) with associated side effects. To provide a more dynamic range of P-gp activity than can be measured with PET, Dr. Kreisl tested several methods of performing 11C-dLop PET in combination with pharmacological inhibition of P-gp. The goal was to determine a safe and reliable method of measuring small increases and decreases in P-gp activity.
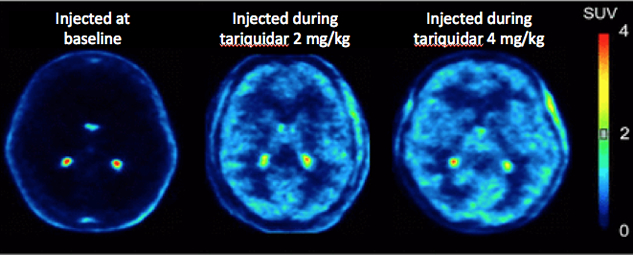
Dr. Kreisl performed PET in healthy volunteers by injecting 11C-dLop in five different conditions: 1) injecting radiotracer under baseline conditions without P-gp inhibition; 2) injecting one hour after IV infusion of tariquidar, 3) injected during IV tariquidar infusion; 4) injecting after oral administration of tariquidar; and 5) injecting after administering disulfiram. Disulfiram was chosen because its metabolites have been shown to inhibit P-gp in vitro.
Neither oral tariquidar nor oral disulfiram increased brain uptake of 11C-dLop. Injecting 11C-dLop during tariquidar infusion, when plasma tariquidar concentrations reach their peak, resulted in brain uptake of radioligand approximately five-fold greater than baseline. Brain uptake was similar with 2 and 4 mg/kg IV tariquidar; however, the lower dose was better tolerated. Injecting 11C-dLop one hour after the tariquidar infusion also increased brain uptake, though higher doses (up to 6 mg/kg) were required, with associated side effects (e.g., nausea and syncope). Brain uptake of 11C-dLop increased fairly linearly with increasing plasma tariquidar concentrations. These results demonstrate that P-gp inhibition is maximal at peak concentrations of tariquidar. Therefore, performing 11C-dLop PET during infusion of low dose of tariquidar allows dynamic measurement of P-gp function while avoiding side effects associated with high doses of the inhibitor. This technique could be used to measure changes in P-gp function in diseases where efflux activity is expected to be decreased (as in Alzheimer’s disease) or increased (as in drug resistant tumors).
William Charles Kreisl
Assistant Professor of Neurology (in the Taub Institute)
wck2107@cumc.columbia.edu