Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Andrew A. Sproul, PhD
 Andrew A. Sproul, PhD
Andrew A. Sproul, PhDI serve as the Director of the Stem Cell and Cellular Models Platform, a proactive resource to facilitate human pluripotent stem cell (PSC) approaches for neurodegenerative disease research in the Taub Institute. We have started working with and/or applied for funding with over 10 research groups within the Taub Institute since our inception in early 2015. We adapt to the needs of each research project and have flexible levels of scientific engagement.
Our main focus is modeling the complexities of Alzheimer's disease (AD) by using genome editing to introduce specific causal and risk mutations into the same genetic background, thus allowing more robust cross comparisons between genotypes and the creation of allelic libraries. Our first library is being constructed in the H9 human embryonic stem cell (hESC) background. hESCs, on average, have a tendency to differentiate more efficiently than iPCSs (induced pluripotent stem cells), and H9, in particular, is attractive due to its ApoE3/E4 genotype. ApoE4 is amongst the strongest risk factors for late-onset AD (LOAD), and may potentiate phenotypes of other risk factors, particularly in neuronal-glial co-cultures.
In order to model familial AD (FAD), as well as establish a baseline for LOAD risk factors, we have used CRISPR/Cas9-mediated genome editing to knockin the APP Swedish mutation (KM670/671NL) into both alleles of the H9 line. The APP Swedish mutation is an autosomal-dominant, 100% penetrant mutation sufficient to cause AD and is commonly used in animal models. FAD and isogenic control neurons are currently available for Taub researchers. Our first LOAD cellular model is being created in collaboration with Dr. Roger Lefort's lab. We have successfully knocked in the EphA1 P460L mutation into the H9 line. This mutation was recently discovered in genome-wide association studies (GWAS) conducted by Dr. Mayeux and colleagues, and correlates with an AD phenotype in a Caribbean Hispanic population.
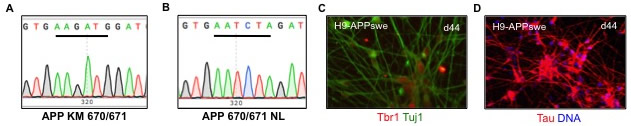 Figure 1. Creation of APPswe/APPswe/ApoE4 hESClines. (A-B) Homozygous knockin of the APPswe (KM670/671NL) into the H9 hESC line was confirmed by Sanger sequencing. (A) shows a WT isogenic control clone and (B) a homozygous knockin mutant. (C-D) The APPswe knockin was differentiated into cortical neurons in monolayer culture. Early-born neurons show the deep layer marker Tbr1 (red, C) and the pan neuronal marker Tuj1 (green, C) and Tau (red, D). |
In addition to creating knockin/knockout lines, we have the capacity to generate iPSCs, including from bio-banked material. iPSCs can be more advantageous than knockins in some cases, such as for risk factors with moderate penetrance that might require additional genetic components to manifest in the patient. We also differentiate both control and AD PSCs into disease-relevant cell types, such as cortical neurons. Finally, we are developing methods to use lentiviruses to overexpress and CRISPSRi to knockdown genes, such as in the case of projects with Dr. Lorraine Clark to study KCNS2, an essential tremor risk gene she identified, and Dr. Andy Teich's group to study ZCCHC17, a potential master transcriptional regulator of synaptic genes in AD.
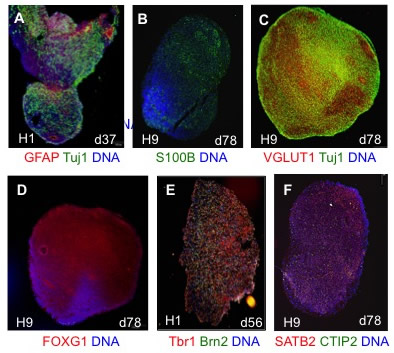 |
| Figure 2. Human cortical spheroid (hCS) differentiation. (A-F) hCSs generated from hESC lines H1 and H9 were cryosectioned and immunostained at the day of differentiation indicated. DNA is shown in blue. (A) hCS cells express the pan-neuronal marker Tuj1 (green) and astrocyte marker GFAP (red) early in the differentiation. (B) By day 60 some cells express the mature astrocyte marker S100B (green). (C) Most cells express the vesicular glutamateric transporter VGLUT1 (red). Neurons are marked by Tuj1 (green). (D) Most cells also express the forebrain marker FOXG1. (E-F) hCSs cells express deep (Tbr1 (red) CTIP2 (green)) and superficial cortical layer markers (Brn2 (green) SATB2 (red)). Note preliminary evidence suggests that layers are intermixed as opposed to the reported layer stratification, at least at the time points analyzed. |
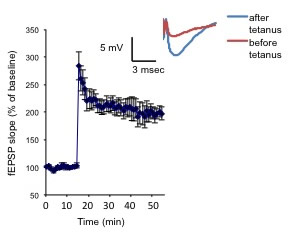 Figure 3. Neurons from PSC-derived hCSs can undergo synaptic plasticity. Potentiation of the fEPSP slope was elicited by a 0-burst tetanic stimulation. The inset shows representative fEPSP before and after tetanus.
Figure 3. Neurons from PSC-derived hCSs can undergo synaptic plasticity. Potentiation of the fEPSP slope was elicited by a 0-burst tetanic stimulation. The inset shows representative fEPSP before and after tetanus.In terms of our own research, I am interested in both technological advances for stem cell–based cellular modeling and further mechanistic insight into AD. For example, perhaps the biggest drawback of human stem cell models is their lack of full-functional maturity. Stem cell derived-cell types such as neurons are more reminiscent of the developing rather than the adult brain. For studying developmental disorders, such as autism, that is advantageous, but for diseases that affect the aged, such as Alzheimer’s, it becomes less optimal. In collaboration with Dr. Ottavio Arancio’s lab, we are developing novel ways to analyze non-adherent 3D cultures called cortical spheroids (hCSs) for electrophysiological function and maturation. hCSs are mixed neuronal-astrocyte cultures that are a kind of floating 'minibrain', and are closer to physiological conditions than monolayer culture. We are essentially treating cortical spheroids as murine brain slice culture, and are able to do assays such as field recordings. We are currently developing strategies to push the neurons within the spheroids into a more mature state to allow the capacity to study LTP (long-term potentiation), a critical component of memory that has not been well modeled in human PSC-derived cultures thus far.
 Andrew A. Sproul and So Yeon Koo
Andrew A. Sproul and So Yeon KooAndrew Sproul, PhD
Assistant Professor of Pathology and Cell Biology (in the Taub Institute)
Director of Stem Cell and Cellular Models Platform
aas2003@cumc.columbia.edu