Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Ottavio Arancio, MD, PhD

Ottavio Arancio, MD, PhD
Research in my laboratory stems from by my life-long commitment to studying mechanisms of synaptic plasticity underlying memory formation. I am interested in the cellular and molecular mechanisms that underlie long-lasting changes of synaptic function in both normal, healthy brains and in the brains of those affected by neurological disorders, in particular Alzheimer's disease (AD). At present we are working on how regulation of gene activation and silencing, post-translational mechanisms, channel opening, intracellular calcium transients and changes in transmitter release machinery might participate in the derangement of synaptic transmission and memory loss. We are answering the following questions:
How do amyloid-beta and tau impair synaptic plasticity and memory? Experiments addressing this question examine amyloid-beta and tau induced modifications in epigenetic and post-translational mechanisms.
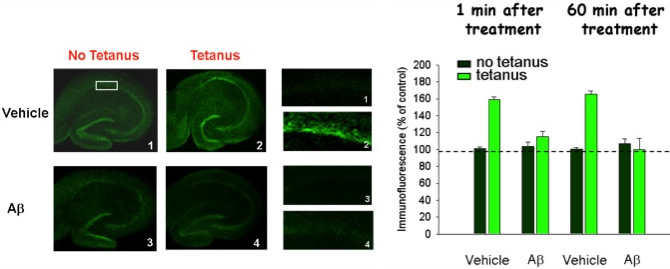
Amyloid-╬▓ impairs phosphorylation of the memory related molecule CREB normally occurring during LTP
Credit: Puzzo et al, J Neurosci-2005Epigenetic mechanisms: We are exploring steps affected at the downstream level of the memory related molecule CREB. CREB plays an important role together with CBP in gene transcription through histone acetylation leading to the loss of chromosomal repression and transcription of genes needed for synthesis of proteins underlying memory formation. Rosita Purgatorio and Mauro Fa' are investigating if reduced histone acetylation follows the CREB inactivation by amyloid-beta and tau. Chromatin changes do not have to be necessarily limited to histone acetylation. As a mechanism which can "lock in" particular states of pathological gene expression in human cells, DNA methylation is an obvious candidate for contributing to the inexorably progression and irreversibility of AD in the middle to late stages of the disease. Additionally, DNA methylation may act early in AD, as some very recent work has shown that the proper regulation of gene expression in memory formation is not only controlled by the transcriptional machinery but also modulated by epigenetics. To this end, Jordano Brito Moreira is currently identifying genes that are differentially methylated following amyloid-beta exposure.
Post-translational mechanisms: SUMOylation is a post-translational mechanism other than phosphorylation involving the covalent attachment of a small 11 kDA protein moiety, SUMO (Small Ubiquitin-like MOdifier), to substrate proteins. Faisal Saeed is investigating if SUMOylation plays a role in learning and memory. He is also investigating whether it is modified following amyloid-beta exposure and if by re-establishing normal SUMOylation one can revert synaptic and cognitive dysfunctions in AD mouse models.
In collaboration with Taub colleague Russell Nicholls, we are investigating the role of the serine/threonine phosphatase, PP2A, in sensitivity and resistance to oligomer-induced behavioral and physiological impairments. Elevated levels of homocysteine and impaired methyl-donor metabolism have been linked to increased AD risk in humans and increased AD-related pathology in cell and animal models. The serine/threonine phosphatase, PP2A, is regulated by site-specific methylation, and misregulated PP2A activity has been proposed to mediate the effects of impaired methyl-donor metabolism on AD. Russell Nicholls generated transgenic mice that over express the PP2A methylesterase, PME-1 or the PP2A methyl transferase, LCMT-1, and found that PME-1 over expression enhanced, and LCMT-1 over expression reduced the physiological and behavioral impairments caused by amyloid-beta and tau. These data support a role for reduced PP2A methylation in increasing AD risk in hyperhomocysteinemic individuals and suggest potential therapeutic effects for increased PP2A methylation in AD treatment or prevention. Moreover, in additional studies, we are currently investigating the involvement of PP2A in traumatic brain injury, another condition that is associated with memory disorders and tau pathology.

Members of the Arancio Laboratory include, top row from left: Andrew F. Teich, Jordano Brito Moreira, and Russell Nicholls. Middle row: Rosita Purgatorio, Jole Fiorito, Deborah Pre', Eva Alba, Faisal Saeed, Vorapin Chinchalongporn, Mitesh Patel, Mauro Fa', and Hanna Berman. Front row, seated: Agnieszka Staniszewski, Ottavio Arancio, and Hong Zhang.
Does amyloid-beta play a critical positive role in synaptic plasticity and memory? Recent research performed in my laboratory has shown that low levels of amyloid-beta similar to those present in the brains of healthy individuals throughout life, enhance synaptic plasticity and memory. Mauro Fa' is continuing these studies by addressing the following questions: can we visualize release of endogenous amyloid-beta and follow its fate in normal physiological conditions? Does release of amyloid-beta from intracellular pools account for the increase in amyloid-beta following activity in the presynaptic terminal? In addition, in collaboration with Taub colleague Andy Teich, we are defining whether the locus of action of amyloid-beta is pre- or post-synaptic. Most importantly, a fundamental question originating from the discovery of a positive function for amyloid-beta is: how does it happen that a molecule performing a positive function gains a new and negative function? To this end, Jordano Brito Moreira is testing whether changes in DNA methylation are responsible for expression of genes that reversibly or irreversibly are responsible for the disease onset.
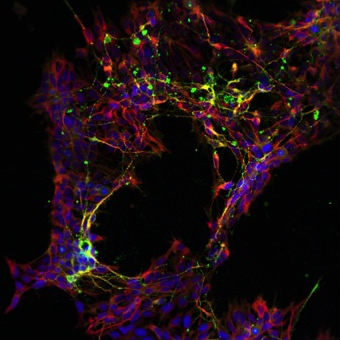
Staining of induced pluripotent stem cell-derived neurons used to investigate neurotransmitter release.It is also by keeping my mind open that I hope to provide an original contribution to research on mechanisms of synaptic plasticity and memory, and particularly to discovering the causes of AD. All this work would be incomplete without the goal to move each project forward to the stage where it not only provides new biological insights but also, when appropriate, serves as the basis for future development of new therapeutic strategies. Such translational research is enhanced by collaborations with Dr. Scott Noggle at the NYSCF and Dr. Don Landry at the Division of Experimental Therapeutics in the Dept. of Medicine of Columbia University. Deborah Pre' and Vorapin Chinchalongporn, in collaboration with Dr. Noggle, are determining whether stem cell technology can reproduce synaptic dysfunction using human derived fibroblasts. With Dr. Landry, Jole Fiorito and Rosita Purgatorio are developing small molecules that activate histones and CREB. These studies should lead to the design of novel therapeutic approaches that might be effective in preventing or delaying the onset of AD and other neurodegenerative diseases characterized by cognitive disorders.
All of the projects in my laboratory are supported by the work of Hong Zhang, Agnieszka Staniszewski, and Li Shi.